Motor protein and cytoskeletal filaments mechanics
Optical tweezers are a powerful tool for studying the activity of motor proteins like kinesin, myosin, or dynein and characterize the mechanical properties of microfilaments and microtubules.
Motor protein activity on microfilaments and microtubules
Optical tweezers are a key tool for understanding how motor proteins like kinesin, dynein or myosin contribute to intracellular transport and organization. Also, the study of the mechanical properties of cytoskeletal filaments such as actin and microtubules is essential for understanding their roles in cellular structure and dynamics. These applications have a strong impact in disease research since studying the effects of mutations or drugs on filament mechanics and motor protein activity is relevant for diseases involving cytoskeletal dysfunctions.
Motor protein activity experiments with optical tweezers
SENSOCELL optical tweezers enable the study of single-molecule motor proteins in isolation, providing insights into their fundamental properties without the interference of bulk effects. Furthermore, thanks to its direct force sensor technology, Sensocell extends the study of motor protein activity from single-molecule assays to experiments in living cells.
A typical single-molecule motor protein experiment consists of different steps:
- Bead attachment: typically, motor proteins are attached to a functionalized microscopic bead. The bead is then trapped by the optical tweezers.
- Filament interaction: the motor protein interacts with its substrate (microtubules for kinesin and dynein, actin filaments for myosin), which can be immobilized on a surface.
- Force and position tracking: as the motor protein moves along the filament, the movement of the bead is tracked with high precision to study the motor’s stepping behavior and force generation.
Microfilaments and microtubules mechanics
Optical tweezers are also highly effective for studying the mechanical properties of cytoskeletal filaments such as actin and microtubules. Potential applications include measuring the filaments stiffness by stretching assays or doing rheological measurements to quantify the viscoelastic behavior of cytoskeletal filaments. Common experimental steps for such assays are:
- Filament preparation: actin filaments or microtubules are polymerized and often labeled with fluorescent markers for visualization.
- Bead attachment: microscopic beads are attached to the ends or specific points along the filaments. These beads are then trapped using the optical tweezers.
- Force application and measurement: Controlled forces are applied using the tweezers, and the resulting displacements are measured with high precision to study the mechanical response of the filaments.
Selected publications:
- Reddy, B., Mattson, M., Wynne, C. et al. Load-induced enhancement of Dynein force production by LIS1–NudE in vivo and in vitro. Nat Commun 7, 12259 (2016).
Application note:
Measurement of the stall force of kinesins in living cells
Video An actin microfilament stretching assay imaged with Epi-FL. Courtesy of Romet-Lemonne and A. Jegou Lab (IJM).
Stall force of kinesin motor protein on microtubules in living cells
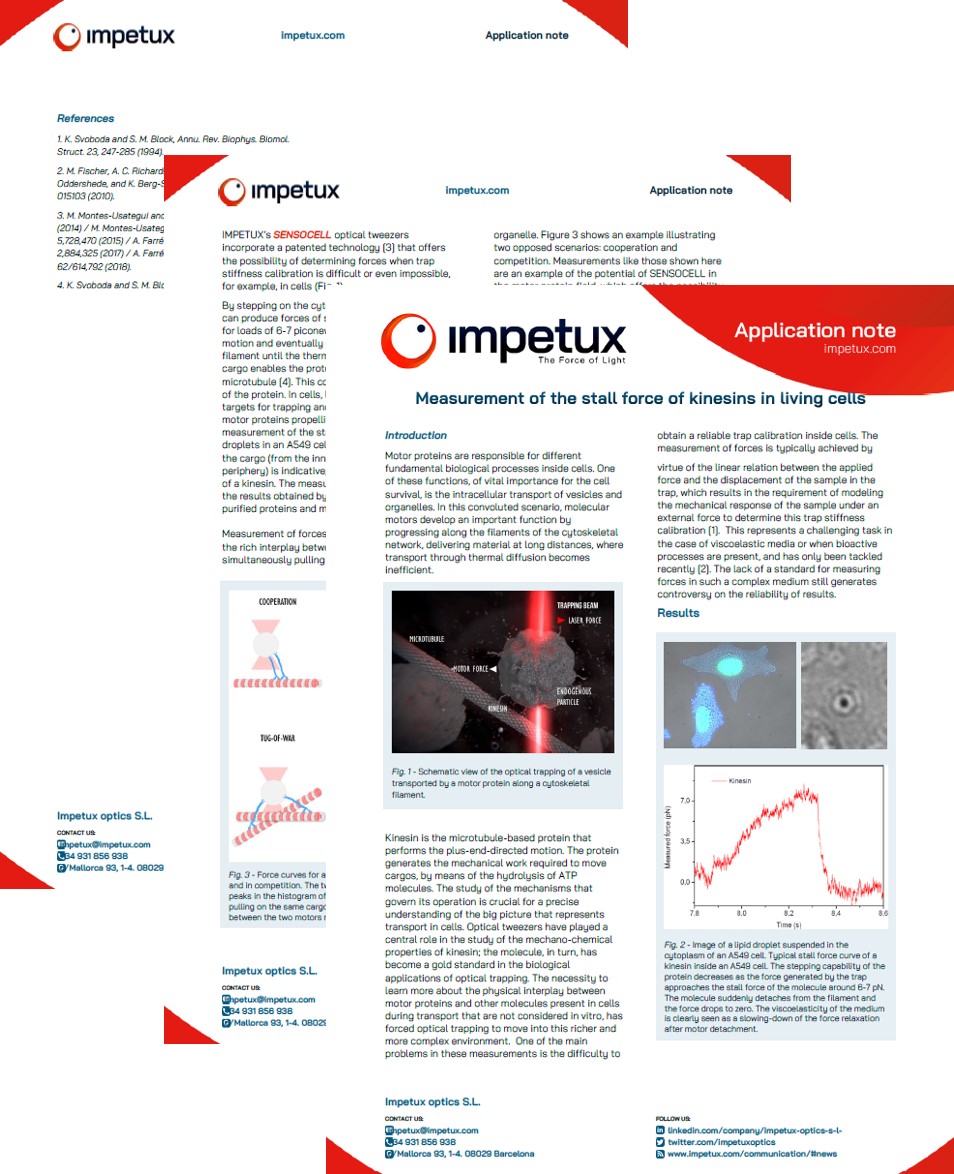
Microtubules motor proteins are responsible for different fundamental biological processes inside cells. One of these functions, of vital importance for the cell survival, is the intracellular transport of vesicles and organelles along microtubules. Kinesin is the microtubule-based motor protein that performs the plus-end-directed motion. The protein generates the mechanical work required to move cargos, by means of the hydrolysis of ATP molecules.
We can use lipid droplets as targets for trapping and analysis of the force of the motor proteins propelling them inside living cells. Fig.1 shows the measurement of the stall force of a kinesin motor protein transporting lipid droplets along a microtubule in a A549 cell.
Related applications:
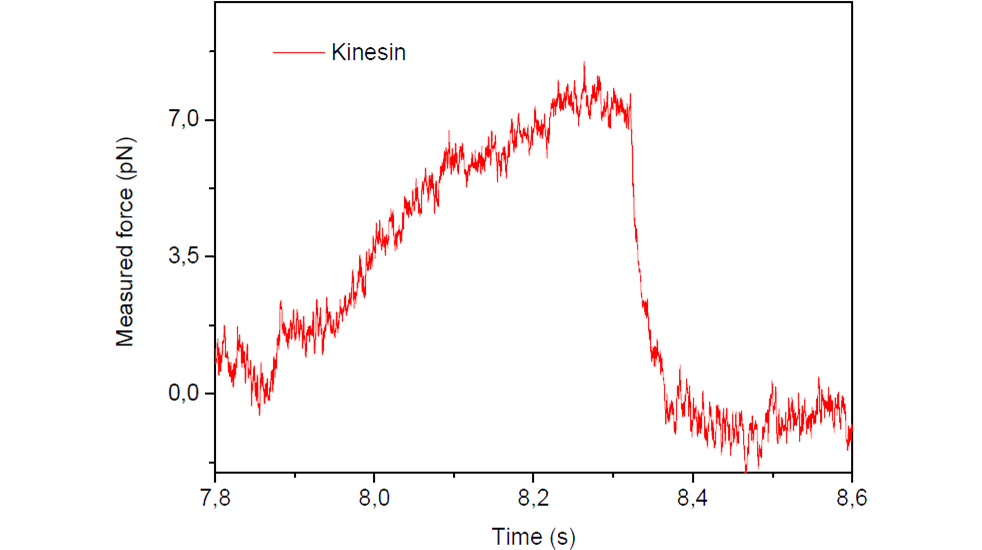
Fig.1 Stall force of a kinesin motor protein carrying a lipid droplet along a microtubule inside an A549 cell. Courtesy of Dr. Mario Montes and Dr. Estela Martín (UB).
Multiple kinesin motor proteins transport on one or two microtubules: cooperation and competition
Measurement of forces inside cells allows exploring the rich interplay between multiple molecular motors simultaneously pulling on the same vesicle/organelle. Here we show an example illustrating two opposed scenarios: cooperation and competition. This is an example of the potential of SENSOCELL optical tweezers in the motor proteins field, which offers the possibility of measuring forces in traditionally difficult or impossible experiments.
Fig. 1 Force curves for a lipid droplet in an A549 cell pulled by multiple kinesin molecular motors in different scenarios: in cooperation and in competition.
Key Concepts
-
- Motor proteins: molecular machines that convert chemical energy, typically from the hydrolysis of ATP, into mechanical work. They move along cytoskeletal filaments, carrying cellular cargo, facilitating muscle contraction, and driving cell motility. Kinesin: moves along microtubules, usually towards the plus end while Dynein typically does it towards the minus end. Myosin moves along actin filaments and is primarily involved in muscle contraction and other cellular movements.
- Microtubules: cylindrical structures made of tubulin protein dimers (alpha and beta tubulin). They are part of the cytoskeleton and play critical roles in maintaining cell shape, enabling intracellular transport, and segregating chromosomes during cell division.
- Microfilaments: also known as actin filaments, are thin, helical polymers of actin proteins. They are flexible and essential components of the cytoskeleton, contributing to cell shape, motility, and division.
- Single-molecule: these studies involve observing and analyzing individual molecules, rather than bulk populations. This approach allows for the detailed examination of the behavior, interactions, and properties of individual molecules without bulk contributions.
Advantages
-
- High precision: SENSOCELL optical tweezers allow for the measurement of very small forces and displacements, providing detailed mechanical characterization at the nanoscale.
- Single-molecule analysis: enables the study of individual motor proteins and filaments.
- Dynamic measurements: facilitates real-time observation under varying mechanical conditions, offering insights into the dynamic properties of filaments and molecular motors.
- Versatility: SENSOCELL can be used in different environments, from in vitro reconstituted systems to in vivo cellular contexts.
- Compatibility: SENSOCELL can be combined with fluorescence imaging techniques such as Epi-FL, Confocal, Super Resolution SIM and TIRF.
Conclusions
SENSOCELL optical tweezers provide a versatile and powerful method for studying motor protein activity and cytoskeletal filaments mechanics, providing precise and quantitative insights into their mechanical properties at the single-molecule level.
Would you like a DEMO?
Download SENSOCELL brochure