Liquid Liquid Phase Separation (LLPS)
Optical tweezers are a powerful tool for studying liquid-liquid phase separation (LLPS) in biomolecular condensates and quantifying their mechanical properties.
Liquid Liquid Phase Separation (LLPS) of biomolecular condensates
Optical tweezers can track changes in the mechanical properties of protein condensates over time, revealing their aging and maturation, transitioning from liquid-like to solid-like states.
Using SENSOCELL optical tweezers to study LLPS in protein droplets
SENSOCELL optical tweezers measure the G’ storage modulus (elastic term) and G” loss modulus (viscous term) of protein droplets, applying oscillatory forces to beads used as probes and tracking their position data. By measuring mechanical properties at different points within a condensate, researchers can identify spatial variations in properties, shedding light on how different regions mature or stiffen differently:
-
- Dual trap rheology assay for interface characterization: this method uses two trapped beads to sandwich the condensate, allowing for detailed measurements of surface tension and shear modulus at the protein droplet interface region.
- Single trap rheology (TimSOM) for bulk characterization: this patented method integrated in SENSOCELL’s workflow, uses a single bead embedded within the protein droplet and a single optical trap to apply forces and measure responses, thus simplifying the experimental setup. The TimSOM active rheology routine automatically computes the complex G modulus (G=G’+iG”) of the material. Sensocell allows measuring the G modulus over 5 orders of magnitude of frequency spectrum.
Rheology of protein droplets and disease research
Rheology assays help understanding how pathological conditions affect the mechanical properties of condensates, potentially leading to diseases like neurodegenerative disorders where protein aggregation and stiffening are common. Additionally, testing potential therapeutic compounds’ effects on the mechanical properties of biomolecular condensates is key for developing treatments that can modulate phase transitions and mitigate diseases related to LLPS phenomena.
Selected publications:
- Sanfeliu-Cerdán, N., Català-Castro, F., Mateos, B. et al. A MEC-2/stomatin condensate liquid-to-solid phase transition controls neuronal mechanotransduction during touch sensing. Nat Cell Biol 25, 1590–1599 (2023)
- Frederic Català-Castro, Santiago Ortiz-Vásquez, Carmen Martínez-Fernández et al. Active microrheology with a single, time-shared laser trap. Biorxive 2023
- Louise Jawerth et al. Protein condensates as aging Maxwell fluids. Science (2020)
Rheology of biomolecular condensates undergoing LLPS
This study led by Michael Krieg’s lab (ICFO) explores the use of TimSOM Rheology integrated in SENSOCELL optical tweezers to measure the frequency-dependent mechanical properties of viscoelastic protein droplets undergoing LLPS. This process is critical for organizing proteins within the cytoplasm of living cells and is tightly regulated to ensure proper cellular functions.
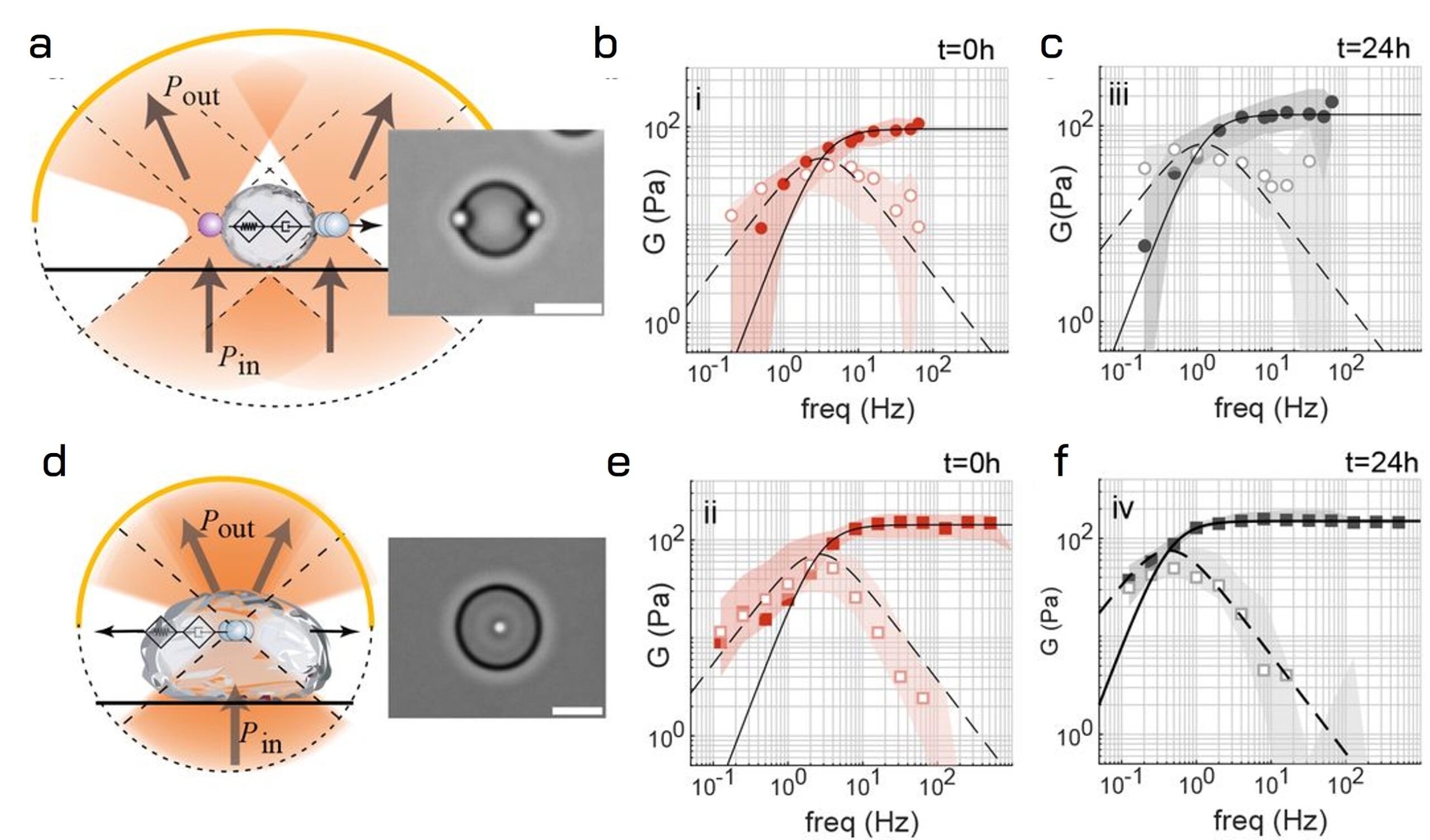
Uncontrolled transitions of these condensates into stiffened states are often linked to diseases. Indeed, increased stiffness correlates with disease severity. The researchers applied TimSOM rheology on MEC-2 condensates, comparing it to the dual trap assay.
Elasticity and viscosity of aging MEC-2 protein droplets
The findings show that both configurations detected a shift in the crossover frequency to lower values after 24 hours of protein droplet formation, indicating maturation. However, the single trap assay (TimSOM) recorded slightly higher stiffness and viscosity, suggesting non-uniform maturation across the droplet. TimSOM effectively resolved the fractional Maxwell behavior of the condensates and revealed a mechanism for rigidity transition across the condensate.
The rheological data of Fig.1 support these findings, showing the storage (G′) and loss (G′′) moduli at different times demonstrating changes in viscosity, stiffness, time constant, and crossover frequency over 24 hours of condensate maturation.
These results confirm that SENSOCELL optical tweezers can accurately measure the viscoelastic properties of aging biomolecular condensates, potentially aiding in drug screening applications.
Related applications:
See the related video example:
Video Dual trap assay rheology for the LLPS characterization of protein droplets.
Key Concepts
-
- Phase Separation: the division of a mixture into distinct regions with different compositions and properties, such as the formation of protein droplets within the cytoplasm.
- LLPS (Liquid-Liquid Phase Separation): a process where a homogeneous liquid solution separates into two distinct liquid phases, often forming biomolecular condensates in cells.
- Biomolecular condensate: a dense, membrane-less compartment formed through LLPS, containing concentrated biomolecules like proteins, crucial for various cellular functions.
- Protein droplet: A type of biomolecular condensate primarily composed of aggregated proteins, often studied to understand their mechanical properties and roles in cells.
- Rheology: the study of the flow and deformation of matter, particularly focusing on the viscoelastic properties of materials like biomolecular condensates.
- G Modulus (Shear Modulus): A measure of a material’s rigidity, indicating how it deforms under shear stress, with storage (G’) and loss (G”) components representing elastic and viscous behavior, respectively.
Advantages
-
- High precision and sensitivity: SENSOCELL optical tweezers offer nanometer-scale spatial resolution and piconewton-scale force sensitivity, essential for detecting subtle changes in the mechanical properties of biomolecular condensates.
- Non-invasive: the technique is relatively non-invasive, minimizing damage to delicate biomolecular structures while allowing for real-time observation of dynamic processes.
- Dynamic measurements: optical tweezers enable real-time tracking of changes in viscoelastic properties of protein droplets, providing insights into the kinetics of phase transitions and maturation processes.
- Versatility: SENSOCELL optical tweezers can be used in various experimental configurations, including single and dual traps, making them adaptable for different experimental conditions, enabling characterization of different regions of the condensates.
Conclusions
SENSOCELL optical tweezers are an invaluable tool for researchers studying liquid-liquid phase transitions in biomolecular condensates. They provide high-resolution, quantitative data on the viscoelastic properties of these structures, helping to unravel the mechanisms underlying their formation, maturation, and role in cellular function and disease.
Would you like a DEMO?
Download SENSOCELL brochure
