Cell nucleus and subcellular mechanics
Study the role of cell nucleus mechanotransduction pathways and how the nucleus mechanical properties regulate cellular activity such as cell migration, development or disease.
The role of intracellular mechanics in mechanotransduction, disease and cell differentiation
Characterize the mechanical properties of intracellular medium like the cell nucleus or the cytoplasm and obtain information of its rigidity, viscosity and elasticity for different cell types and conditions. Investigate how diseases like cancer or laminopathies affect nuclear mechanics and how these influence and reflect cellular differentiation and development.
Cell nucleus indentation with Stress-Relaxation assays
Use Sensocell optical tweezers to perform cell nucleus indentation experiments inside living cells, embryos or organisms using internalized microspheres or subcellular structures such as lipid droplets as probes. Stress-relaxation tests involve applying a constant deformation to the nucleus and observing how the force (or stress) relaxes over time:
- Indenting and holding: the bead or the lipid droplet is trapped and used to indent the nucleus to a certain depth and held at that position.
- Observing relaxation: over time, the force required to maintain the indentation decreases as the nucleus relaxes. This force relaxation is recorded.
- Analyzing relaxation data:
- Viscoelastic properties: the relaxation behavior provides insights into the viscoelastic properties of the nucleus. A common approach is to fit the relaxation data to a viscoelastic model, such as the Maxwell or Kelvin-Voigt models.
- Time constants: these models provide time constants that describe how quickly the nucleus relaxes, which is related to its viscosity and elasticity.
Creep tests for intracellular mechanics experiments
Creep tests involve applying a constant force to the probed material and observing how the deformation (strain) evolves over time. These tests are particularly useful for understanding the long-term viscoelastic behavior of the nucleus or the cell cytoplasmic medium.
Selected publications:
- V. Venturi et al., The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science (2020)
- Frederic Català-Castro et al.. Direct Force Measurements of Subcellular Mechanics in Confinement using Optical Tweezers. J. Vis. Exp. (2021)
- Frederic Català-Castro, Santiago Ortiz-Vásquez, Carmen Martínez-Fernández et al. Active microrheology with a single, time-shared laser trap. Biorxive 2023
- De Lope-Planelles, A., González-Novo, R., Madrazo, E. et al. Mechanical stress confers nuclear and functional changes in derived leukemia cells from persistent confined migration. Cell. Mol. Life Sci. 80, 316 (2023)
Cell nucleus indentation in isolated or cultured cells
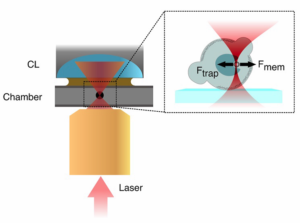
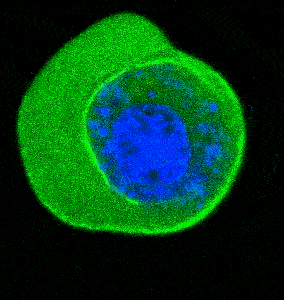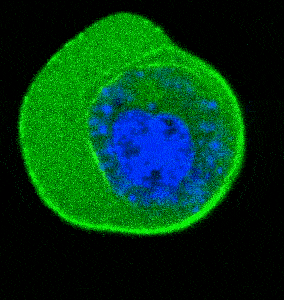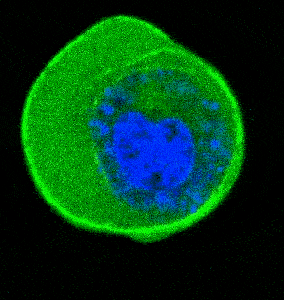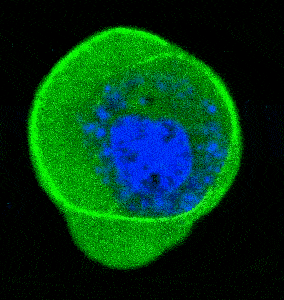
The following images and videos are courtesy of the lab of Dr. Verena Ruprecht (CRG) in collaboration with Dr. Stefan Wieser and Dr. Michael Krieg (ICFO). This example is part of a work (Science, 2020) dealing with the role of nucleus mechanotransduction pathways to control cell deformation behavior and cell migration plasticity. The authors used SENSOCELL optical tweezers to optically trap and manipulate 1 μm fluorescent beads and indent the nuclei of stem cells extracted from zebrafish embryos. The beads had been previously internalized into the 1-cell early embryos. For the experiments, the authors used automatized Stress-Relaxation routines consisting in predefined back and forth trap motions.
Fig. 1 Top: experimental sketch: injected microspheres are optically trapped to indent the nuclei of suspended and confined cells from Zebrafish embryos. Bottom: confocal video of a cell nucleus indentation experiment.
Optical trap time-position and time-force curves
Before launching the Stress-Relaxation routine, the optically trapped bead is some microns away from the nucleus membrane. When we launch the routine, the optical trap moves towards the nucleus at a certain speed until it reaches the nucleus membrane and the indentation takes place. At this point, the optical trapping force increases from zero and gives rise to a force peak. The indentation depth depends on the predefined optical trap trajectory. In this case, the routine was programmed in such a way that the optical trap remained indenting the cell nucleus for 10s. During these 10 seconds, we observe a relaxation in the force signal. Next, the optical trap goes back to its original position and the recorded force drops down to zero. Figure 2 shows the optical trap time-position and time-force curves for a single cell nucleus indentation experiment.
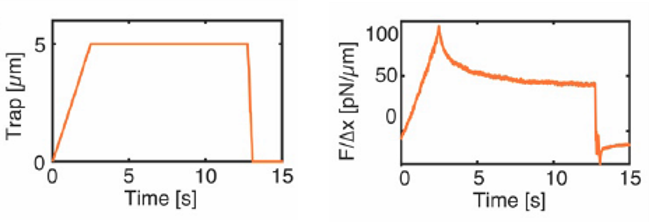
Fig. 2 Left: time-position curve of the optical trap for one of the nucleus indentation experiments. Right: force profile during the nucleus indentation experiment.
Characterization of the cell nucleus mechanical properties in suspended and confined cells
Below, we can see some results obtained from an exponential-decay fitting of such force relaxation curves for cells in suspension and cells confined in microchambers. From these fittings the authors obtain the characteristic relaxation time and the static force given by the residual stress applied onto the nuclear membrane for both conditions.
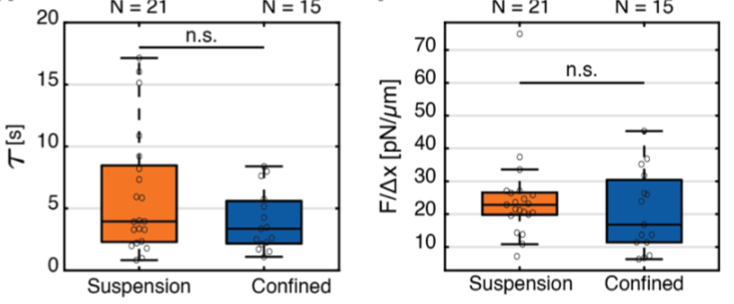
Fig. 3 Left: Characteristic decay time of the force relaxation curve for cell nucleus indentation experiments carried out in suspended and confined cells. Right: measurements of the cell nucleus stiffness for both suspended and confined conditions.
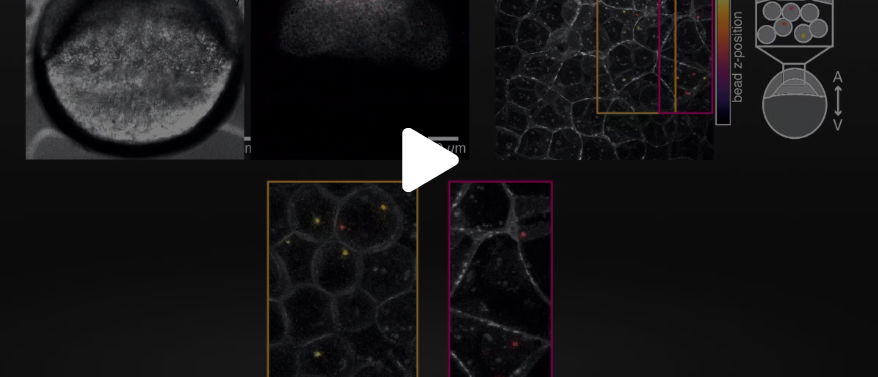
Cell nucleus indentation inside tissues and living organisms (C. elegans & Zebra fish)
This is an example of a cell nucleus indentation experiment performed inside the intestinal cells of a living C. elegans organism (Biorxive 2023). Courtesy of Michael Krieg’s lab (ICFO). In this work the researchers show how endogenous lipid droplets are excellent probes to perform intracellular mechanic experiments with Sensocell optical tweezers, including nucleus deformation by stress-relaxation assays.
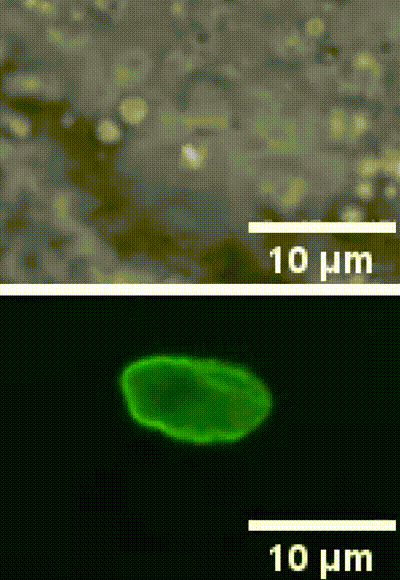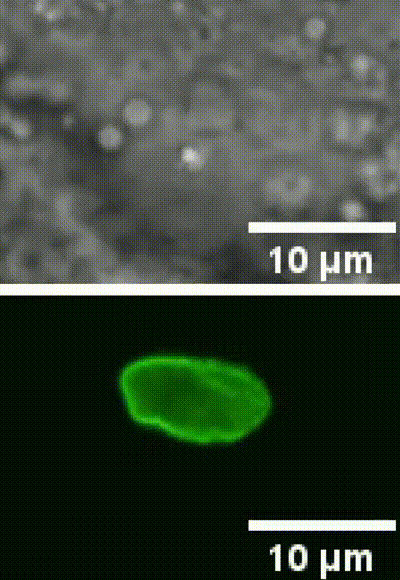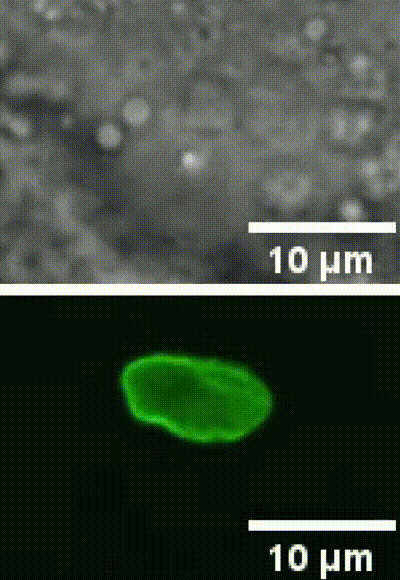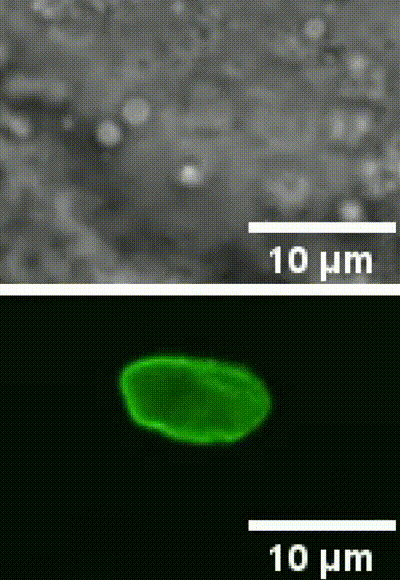
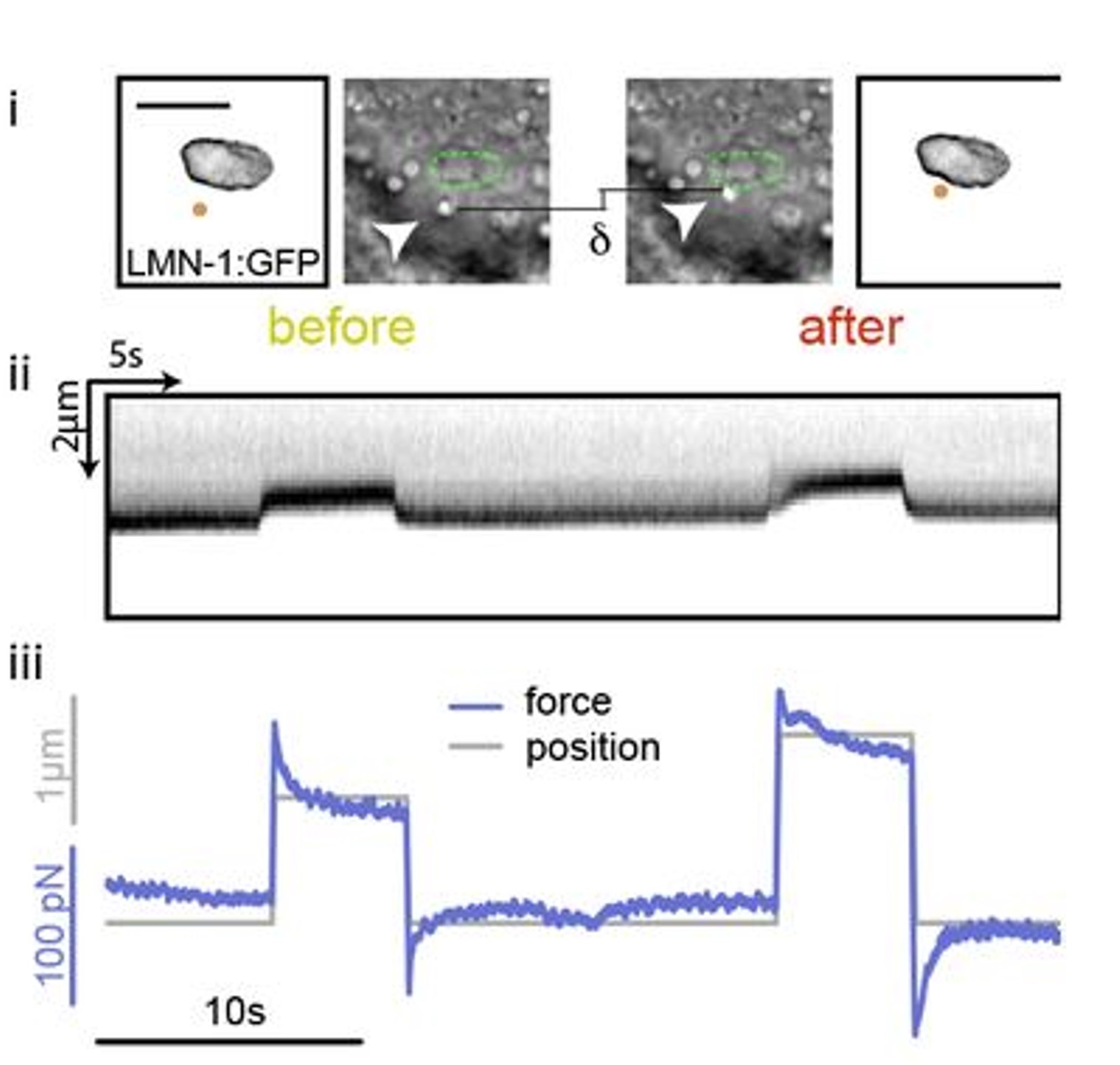
Fig. 2 (i) GFP fluorescence and bright field images showing nuclear deformation with a trapped lipid droplet in an intestinal epithelial cell inside a C.elegans organism. δ indicates the deformation of the nucleus during the test, arrowhead points to the trapped lipid droplet. Scale bar = 2µm. (ii) Kymograph of two consecutive step indentations of an intestinal nucleus using a lipid droplet as force probe. (iii) Force and displacement during the same step indentation protocol.
Video Confocal video of a cell nucleus indentation assay performed inside a Zebra fish embryo (5 hours after fertilization). In this case, the authors used internalized beads (red) as handles. The trapped beads can be moved to different locations within the cell.
Force transmission in a dual trap cell nucleus indentation assay
The following images and data illustrate a dual-trap cell nucleus indentation experiment conducted using a pair of 1 μm beads internalized in zebrafish stem cells. Each bead indents the cell nucleus sequentially at different times. The system continuously records the force and position data of both traps, enabling the detection of any force transmission between them. While control cells do not exhibit force transmission during nucleus indentation, cells treated with Latrunculin A for actin depolymerization show a distinct force propagation signature. This indicates that actin plays a crucial role in stabilizing the position of the cell nucleus within the cell.
Related applications:
Fig. 1 of a dual trap cell nucleus indentaion assay with Sensocell optical tweezers
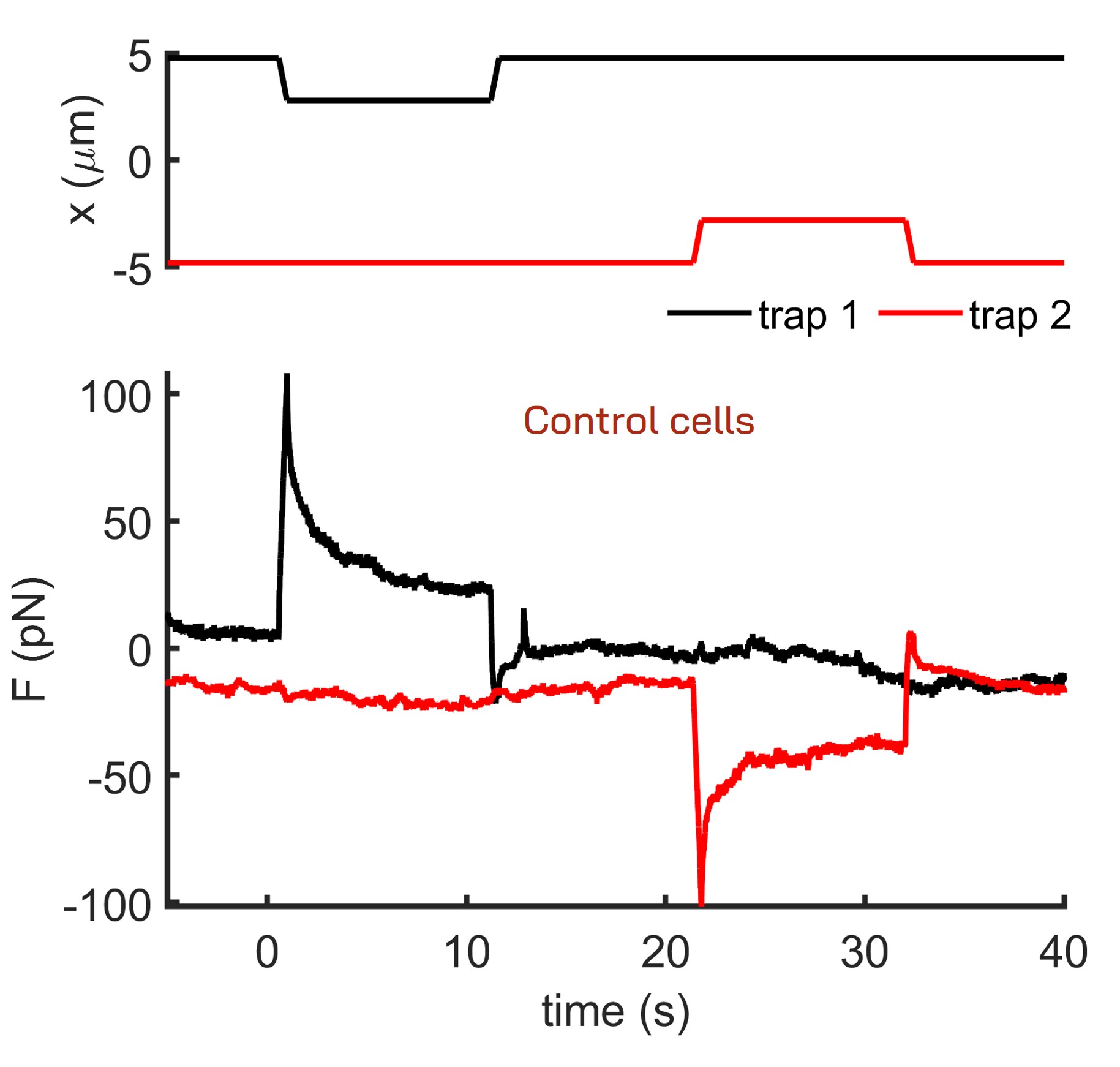
Fig. 2 Time-position and time-force curves obtained during a dual trap cell nucleus indentation test of control cells.
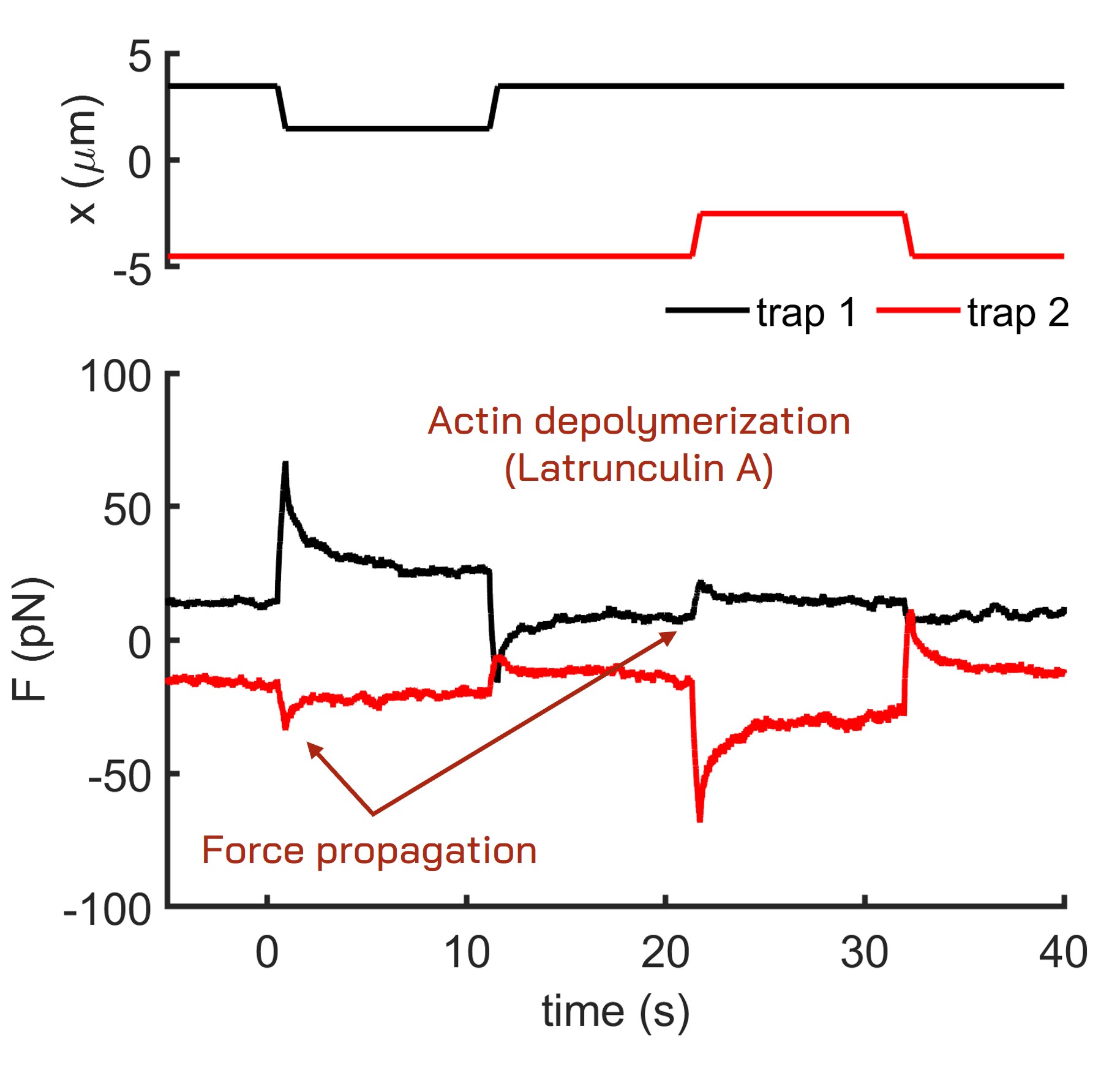
Fig. 3 Force propagation in an equivalent indentation assay with cells treated with Latrunculin A.
Protocol for subcellular mechanics experiments with optical tweezers
Here, the authors present a protocol to investigate the intracellular mechanical properties of isolated embryonic zebrafish cells in three-dimensional confinement with direct force measurement by an optical trap.
Introduction / Single-cell Preparation and Staining / Optical Trapping (OT) Chamber Spacing and Optical Tweezers Start-up / Alignment of the Optical Force Sensor / Performing the Nucleus Indentation Experiments / Results: Measurement of the Forces and Material Properties of the Cell Nucleus Inside Zebrafish Embryo / Conclusion. See full video here.
How to perform Creep and Stress-Relaxation Tests with Sensocell
In this video example, the vacuole of a yeast cell was optically trapped and used to probe the cellular medium in two ways:
First, we perform a Creep test: the force feedback routine (force clamp) tracks the cell’s vacuole movement while a constant force of 5 pN is applied on it. The data from the plot panel show force and position signals (orange and blue lines respectively). When the force clamp is applied, the position signal follows the classical behaviour expected for a viscoelastic medium. When the force clamp is stopped, the force signal relaxes back to zero (negative values) and the position signal abruptly shifts to zero.
Next, we launch a Stress-Relaxation test using the same trapped vacuole as a probe. In this case, we apply a trajectory to the optical trap to move it 300 nm to the right in 0.1 s while the applied force is tracked. We can observe a peak in the force when the trap position is shifted followed by a relaxation of the force while the trap remains in a fixed location.
See other video examples:
Key Concepts
- Elastic Modulus (E): a measure of the stiffness of the nucleus, derived from the force-indentation data.
- Viscoelasticity: describes materials that exhibit both viscous and elastic characteristics when undergoing deformation.
- Indentation Depth: the depth to which the bead indents the nucleus provides information about the mechanical resistance of the nuclear envelope and the underlying nuclear structure.
Advantages
- Precision: high-resolution measurements allow for detailed characterization of nuclear mechanics.
- Non-destructive: these experiments can be minimally invasive, preserving cell viability and function.
- Temporal resolution: Stress-relaxation tests provide dynamic information about nuclear mechanics over time.
- Direct measurements: our calibration-free force sensor enables intracellular mechanics experiments in an easy and straightforward manner.
Conclusions
In summary, Sensocell optical tweezers is a powerful tool for measuring the mechanical properties of the cell nucleus. Indentation experiments provide data on stiffness and elasticity, while stress-relaxation tests reveal viscoelastic properties, offering comprehensive insights into nuclear mechanics.
Would you like a DEMO?
Download SENSOCELL brochure